Free Shipping
From €130 without VAT *SECURE PAYMENT
6 forms of paymentCustomer Support
Phone +34 963627900Warranty
Customer satisfactionMUST-HAVES! BACK TO THE CLINIC
PRODUCTS OF THE WEEK
UREA CREAMS
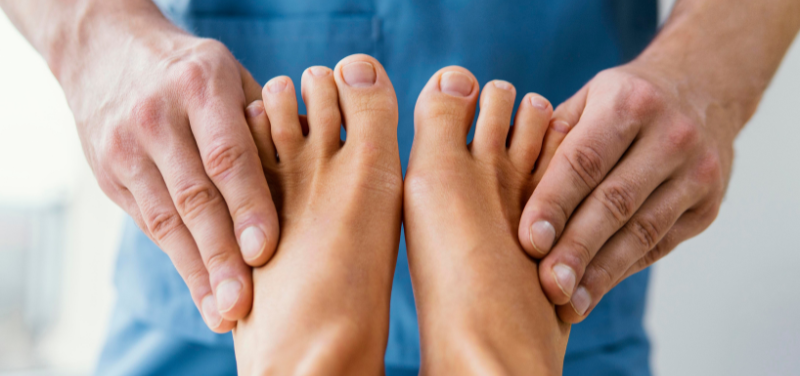

Delve into the science of foot cares
HERBITAS: PODIATRY AND MEDICAL SUPPLIES
Laboratorios Herbitas was created in 1988. The company has grown and consolidated itself since its creation as a national reference in the podiatry and medical sector, with numerous innovations and creations that have been introduced in this field.
Company
Reason for being
Our vocation is the permanent research of the customers' satisfaction and the development of our professionals as leader in podiatry and medical supplies. We encourage innovation in a sustainable and ethical environment through excellence in service.
Vision
Herbitas bets on the future expanding its facilities. Improving the technological part and training the team to give the best service to our customers.
Why do you have to buy podiatry and medical supplies in Herbitas?
Why do you have to buy podiatry and medical supplies in Herbitas?
The reason is that Herbitas has an extensive catalogue, the quality of our medical and podiatry products and our continuous innovation. Moreover, the experience accumulated over all these years make Herbitas your perfect supplier for all types of material and medical equipment you need. We cover several fields such as podiatry, physiotherapy, dental, laboratory, pharmacy, esthetics, tattoo and so on.

Secure Payment
Secure Payment
The payment methods are various: VISA, PAYPAL, BANK DRAFT, TRANSFER. All these payment methods make the customers feel comfortable and secure when it comes to choose the form of payment that best suits them. Always with the peace of mind of trusting their data to a company that complies with the RGPD.
Customer Service
Customer Service
The customer care and attention are in the DNA of Laboratorios Herbitas. This company was born and is still a familiar business and its philosophy is spread through all the team. We look for the client to trust us by creating a relationship that is maintained over time. Laboratorios Herbitas strives to provide the customer with good care and better service. The satisfaction of our customers is the reason on which all our efforts revolve.
Flash offers
Flash offers every Monday
Enjoy the best offers every week where you can find products at a reduced price. Subscribe to our newsletter or download the Herbitas APP to be informed anytime and anywhere. Do not let them get away!